LLNL’s Basic Energy Sciences (BES) program seeks to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels.
Office of Science supported research at LLNL includes efforts in the areas of nanoscale biomaterials science, mesoscale materials for catalysis, materials degradation, quantum information science, and the development of open-source software tools for computational materials science. This funding also supports LLNL’s national security mission through research on synchrotron radiation and neutron sources at national scientific user facilities.
LLNL BES Programs
Design, Synthesis, and Assembly of Biomimetic Materials with Novel Functionality
Our goal is to develop a quantitative physical picture of macromolecular organization and its relationship to function, emphasizing biomimetic membrane transport systems that combine designed sequence polymers with engineered nanoscale pores. We focus on:
- Applying physicochemical principles of peptoid assembly and nanopore incorporation.
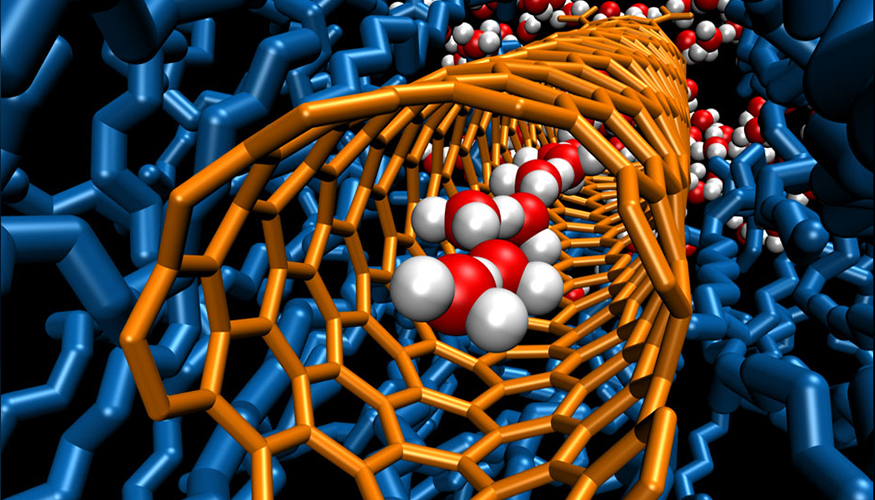
- Incorporating carbon nanotube (CNT) porins into peptoid membranes and elucidating structure of these assemblies.
- Exploring other biomimetic functionality of peptoids and CNT porins.
Quantum Probes of the Materials’ Origins of Decoherence
We are working to develop an understanding and control over sources of materials-induced decoherence in materials, which is required to realize more than an order-of-magnitude improvement in solid-state qubit and quantum sensor coherence times.
Understanding Degradation Mechanisms of Aminopolymers Used in Direct Air Capture
Development of chemistry- and physics-based understanding of fundamental degradation mechanism and kinetics will pave the way for novel mitigation paradigms and reduce uncertainty associated with sorbent lifetime and cost. Key questions driving the project:
- What are the key reaction mechanisms and energy barriers for oxidative degradation?
- How does the structure of the oxidized aminopolymer affect performance for CO2 capture?
- How do co-adsorbates affect observed reaction mechanism and degradation rate?
Center for Predictive Simulation of Functional Materials
We are developing, applying, validating, and disseminating parameter-free methods and open-source codes to predict and explain the properties of functional materials for energy applications. Research foci include:
- Phase transitions and equation of state properties of high-pressure systems.
- Quantum Monte Carlo-based first principles simulations.
- Defects in transition metals and semiconductors.
- Development of pseudo-potential database for Quantum Monte Carlo (QMC).
Learn more about the Center for Predictive Simulation of Functional Materials
Center for Non-Perturbative Studies of Functional Materials under Non-Equilibrium Conditions (NPNEQ)
Researchers from LNLL, Lawrence Berkeley National Laboratory, and SLAC National Accelerator Laboratory are using an experiment–theory approach to develop open-source software that will facilitate fundamental advances in materials science. Project objectives include:
- Augmenting and exploiting the capabilities of the Qbox code.
- Harnessing the Department of Energy's high-performance computing systems.
- Computationally modeling quantitative and non-perturbative responses of functional materials.
Energy Frontier Research Centers (EFRCs)
The Center for Enhanced Nanofluidic Transport (CENT)
CENT addresses emerging and compelling gaps in our scientific knowledge of fluid flow and molecular transport in extremely narrow nanopores to develop the basic science for the next generation of energy-efficient separation technologies.
Integrated Mesoscale Architectures for Sustainable Catalysis (IMASC)
IMASC addresses the challenge of designing and perfecting atom- and energy-efficient synthesis of revolutionary new forms of matter with tailored properties. LLNL has been working to stabilize the catalytic activity of a nanoporous NiCu alloy catalyst for nonoxidative dehydrogenation of ethanol, which generates hydrogen as a byproduct while avoiding separation of water. In pursuit of this project, we have:
- Developed nanoporous NiCu dilute alloy catalyst for nonoxidative ethanol dehydrogenation.
- Followed dynamic changes of the surface morphology and composition during activation and deactivation with in situ and ex situ x-ray photoelectron spectroscopy and electron-microscopy.
- Stabilized catalytic activity of dilute NiCu alloys by generating a kinetically trapped Ni2+ subsurface state.
BES Highlights
Strong Water/Ion Coupling in 1.5-nm Diameter Carbon Nanotube Porins
LLNL’s research in ion transport showed very strong electroosmotic coupling in 1.5-nm-diameter carbon nanotube porins (CNTPs) that manifests in the unusual two-thirds power-law conductance scaling. Experiments and computer modeling show that high slip flow in carbon nanotube pores produces strong coupling between water and ion motion. Findings include:
- CNTPs show weakly cation-selective ion transport following two-thirds power law.
- Modeling indicated that this scaling originates in the high slip conditions at the CNTP wall.
- The predicted figure of merit for the CNTP-based, low-voltage electroosmotic pumps could be 10 times higher than state-of-the-art pumps.
Strong Differential Monovalent Anion Selectivity in Narrow Diameter Carbon Nanotube Porins
We have demonstrated monovalent anion transport through 0.8-nm-diameter carbon nanotube porins (CNTPs) with strong selectivity, where the permeation rates vary by up to two orders of magnitude. The dehydration energy penalty dominates the anion selectivity of carbon nanotubes and leads to unusual differential anion selectivity. Findings include:
- CNTPs show a strong anion-selective transport following the order of SCN− > I− > Br− > Cl−.
- Removal of negative charges from entrance increases the permeability only slightly.
- Simulations show that the permeability values have strong correlations to ion hydration energy.
Carbon Nanotube Porins Provide Robust “Skin-Like” Protection for Nanowire Biosensors
LLNL demonstrated functional carbon nanotube porin (CNTP) membranes that protect Si-nanowire-based biosensors. This is the first demonstration of a protective membrane that exploits high-proton permeability and strong size selectivity of 0.8-nm CNTPs to protect pH sensors from fouling by a variety of biological fluids. Results include:
- Embedding CNTPs into a lipid layer coating a nanowire biosensor surface enables construction of protective skin-like membranes.
- CNTPs allow protons to reach the sensor surface and are much too small to allow the protein to go through the pores. Lipid bilayer also strongly resists protein adsorption.
- Devices protected by CNTPs show full functionality after 3 days of exposure to solutions of bovine serum albumin, milk, and blood plasma that irreversibly foul unprotected devices.

Tony van Buuren
Lab Program Coordinator for BES at LLNL
vanbuuren1 [at] llnl.gov (vanbuuren1[at]llnl[dot]gov)